Main Content
Changing lives: A lasting-legacy of effective design
Rarely in the history of medicine has a discovery delivered such a dramatic and immediate effect as that of insulin.
In 1922 a Canadian hospital ward was full of very sick children. In diabetic comas, their prognosis was extremely poor – but as a medical team moved around the ward injecting the children with a trial of insulin, they awoke one-by-one from their coma… and the rest, as they say, is history. Or is it?
Although the medical breakthrough has given hope and life to millions of diabetics around the world in the hundred years since, a diabetes diagnosis still remains life changing and fraught with risk.
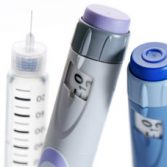 Being able to manage diabetes and survive with it, can mean daily injections for many. Learning how to use an insulin treatment device, overcoming a fear of needles, managing the discomfort on a daily basis and ensuring the correct dosage are all hugely significant factors in the quality and outlook of life for diabetics.
Being able to manage diabetes and survive with it, can mean daily injections for many. Learning how to use an insulin treatment device, overcoming a fear of needles, managing the discomfort on a daily basis and ensuring the correct dosage are all hugely significant factors in the quality and outlook of life for diabetics.
That was the start point for an ambitious design brief instigated over a decade ago. A design that won the DBA Design Effectiveness Award Grand Prix in 2009 and which still continues to have a transformative impact around the world today.
The original brief was clear; to create a new, disposable pen injector for the delivery of insulin that would offer significant improvements over all other disposable insulin devices on the market in terms of comfort, safety and ease of use for patients.
Launched in 2007, the solution was SoloSTAR. Created by Sanofi Aventis and agency DCA Design International through an evidence-based engineering design process, the injector pen was capable of delivering a single insulin dose 30% higher than other competitor devices.
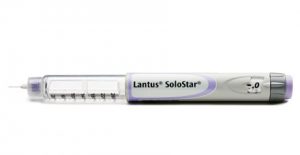 Significantly, SoloSTAR’s innovative design also enabled it to deliver the lowest injection force in its class (by a whopping 30%) providing a much smoother injection experience for patients. Being such a key factor in patient comfort, it’s not surprising that 7 out of 10 patients reported a preference for the injection force of the SoloSTAR device and in 2008 it accounted for 41% of all growth in the global injectable insulin market.
Significantly, SoloSTAR’s innovative design also enabled it to deliver the lowest injection force in its class (by a whopping 30%) providing a much smoother injection experience for patients. Being such a key factor in patient comfort, it’s not surprising that 7 out of 10 patients reported a preference for the injection force of the SoloSTAR device and in 2008 it accounted for 41% of all growth in the global injectable insulin market.
With its discrete and appealing form to negate stigma attached to public use, and a design that carefully considers risk and accessibility factors for diabetics, SoloSTAR has delivered important safety and usability advantages for patients – especially those with visual and physical impairments. Medical professionals also welcomed SoloSTAR and the reduction in time needed to teach patients how to use it. As one diabetes nurse noted “we can teach more patients and teach them more quickly. That is a huge advantage.”
As the prevalence of diabetes grows with 463 million adults living with diabetes in 2019 and set to rise to 700 million by 2045, the continued innovation of drug delivery systems is paramount to maintaining functional lives for millions across the globe.
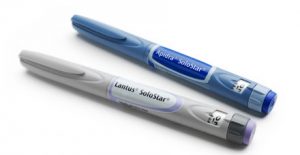 When launched in 2007, Sanofi Aventis and DCA initially developed two variations of the SoloSTAR pen for two insulin types; Lantus (basal insulin, typically taken once a day) and Apidra (taken with meals, 3 or more times a day) and over a decade on, the SoloSTAR device continues to be sold in large volumes globally today.
When launched in 2007, Sanofi Aventis and DCA initially developed two variations of the SoloSTAR pen for two insulin types; Lantus (basal insulin, typically taken once a day) and Apidra (taken with meals, 3 or more times a day) and over a decade on, the SoloSTAR device continues to be sold in large volumes globally today.
Testament to the effectiveness of the collaboration and the design, is that there are now over ten variants in the SoloSTAR range, each using a similar mechanism to the original device, but optimised for purpose.
“When developing the Toujeo SoloSTAR and Toujeo Max SoloSTAR injectors which are used for concentrated insulin”, reflects DCA Design International’s Rob Veasey, “we adjusted the mechanism to provide an even lower injection force.”
Although other competitors have risen to the challenge with attempts to mimic the SoloSTAR mechanism, Sanofi’s SoloSTAR range continues to be an important product and Sanofi remains one of the world leaders of the market.
 The continued effective collaboration between Sanofi and DCA has included the development of a highly competitive new pen injector for the Indian market, where there are rising cases of diabetes. The success of this device has subsequently led to the creation of a variant for other global markets, the AllStar Pro reusable pen injector which launched in 2017.
The continued effective collaboration between Sanofi and DCA has included the development of a highly competitive new pen injector for the Indian market, where there are rising cases of diabetes. The success of this device has subsequently led to the creation of a variant for other global markets, the AllStar Pro reusable pen injector which launched in 2017.

“Being able to demonstrate the effectiveness of our design work is the lifeblood of a consultancy like ours,” says Veasey, “what really matters is being able to give demonstrable examples of how our designs achieved commercial success for a client – that’s fundamentally important for our business.
Having winning case studies such as SoloSTAR helps us evidence the power of design and communicate its strategic value in a persuasive way. SoloSTAR remains a great example of successful design and the product continues to be a high selling drug delivery device across the world.”
The SoloSTAR Pen Injector won the DBA Design Effectiveness Grand Prix Award in 2009. Read the full case study here.
DBA Design Effectiveness Awards: Open for entries
 Find out more about the DBA Design Effectiveness Awards and how to enter: visit effectivedesign.org.uk and sign up for updates.
Find out more about the DBA Design Effectiveness Awards and how to enter: visit effectivedesign.org.uk and sign up for updates.
Image credits:
Owen Beard | Unsplash
Sanofi Aventis and DCA Design International